Never miss an update from Universidad de Alicante
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
The Institute of Organic Synthesis and the Institute of Water and Environmental Sciences, belonging to the University of Alicante, have jointly developed an extractant mixture formed by the combination of a process ionic liquid (TSIL) and an ionic liquid (IL) that allows the selective and efficient extraction of metals of the f series of the periodic table (lanthanides and actinides) with respect to other metals of the s, p and/or d series. This technology is characterised by the fact that the extractant mixture can be reused in new extraction cycles without losing effectiveness, which represents a great advance in sustainability and environmental protection.This novel formulation can be applied industrially in areas such as mining, nuclear chemistry, nuclear medicine and nuclear waste treatment.Companies interested in acquiring this technology for commercial exploitation through patent licensing agreements are being sought.
TECHNICAL DESCRIPTION
In order to solve the problems described above, an extractant mixture consisting of a TSIL process ionic liquid (see chemical formula in Figure 1) dissolved in an IL ionic liquid (see chemical formula in Figure 2) has been developed.
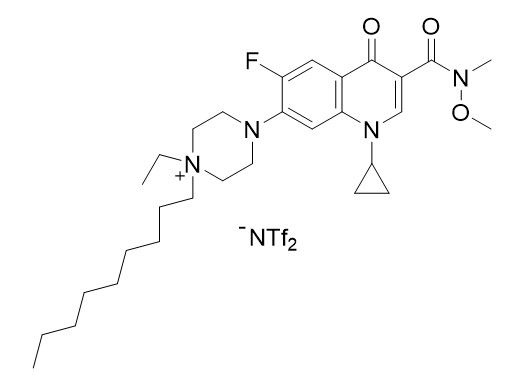
Figure 1: TSIL compound.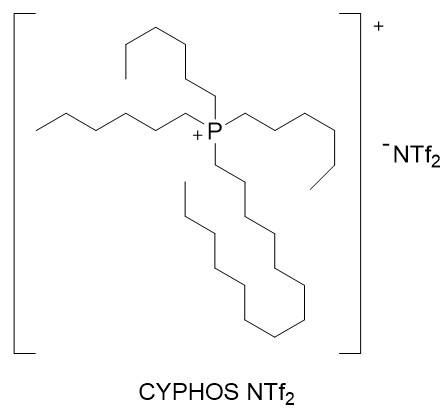
Figure 2: Ionic liquid IL (CYPHOS NTf2).
This extractant mixture is characterised by the fact that it allows the selective liquid-liquid extraction of f-block metals of the periodic table (lanthanide and actinide series) in samples containing other metals of the s-, p- and/or d-series.
The procedure to prepare the above TSIL compound is carried out in a very simple way from the antibiotic ciprofloxacin using conventional functional group transformations. The synthesis of this compound takes place in three steps under mild reaction conditions (room temperature to 0°C, atmospheric air pressure, etc.).
Step 1: N-alkylation reaction of ciprofloxacin with 1-bromoethane in the presence of excess N,N-diisopropyl(ethyl)amine to generate the following intermediate (see Figure 3) in 88% yield:
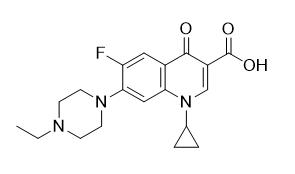
Figure 3: Reaction intermediate.
Step 2: Amidation reaction of the above compound using an excess of N,O-dimethylhydroxylamine in the presence of thionyl chloride to give the following Weinreb's amide (see Figure 4) in 86% yield:
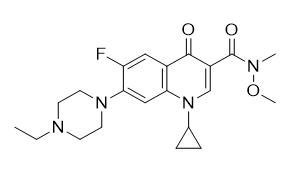
Figure 4: Weinreb's amide.
Stage 3: Alkylation reaction of the above compound by the addition of excess 1-iodonononane and subsequent ion exchange with lithium bis(trifuromethanesulfonyl)amidide to obtain the above TSIL molecule (see Figure 1) in 92% yield.
On the other hand, the procedure for the selective extraction of f-block metals in a sample containing f-block metals together with other s-, p- and/or d-series metals are as follows:
1) Prepare the extractant mixture (B) consisting of the compound TSIL from Figure 1 and the ionic liquid CYPHOS NTf2 from Figure 2.
2) Place the sample to be separated (A) containing one or more f-block metal(s) and other metals belonging to the s-, p- and/or d-series into a test tube. Adjust the pH to 6.
3) Add the extractant mixture (B) to the sample to be separated (A).
4) Shake for at least three minutes.
5) Wait until two liquid-liquid phases of different densities are differentiated.
6) Separate the two phases, the organic phase being the one corresponding to the extractant mixture (B) which includes the metal (or metals) of block f initially contained in the sample to be separated (A) (see Figure 5).
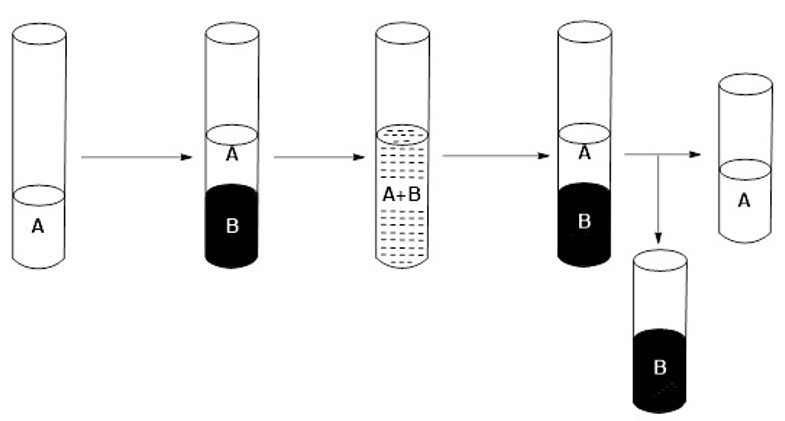
Figure 5: Schematic of the selective extraction process.
7) Add to the recovered organic phase (B) an acidified solution at pH=0.5 (C).
8) Stir.
9) Wait until two liquid-liquid phases of different densities are differentiated.
10) Separate the two phases, where the organic phase corresponds to the metal-free extractant mixture (B) and the aqueous phase (C) containing the metal(s) of f-block (see Figure 6).
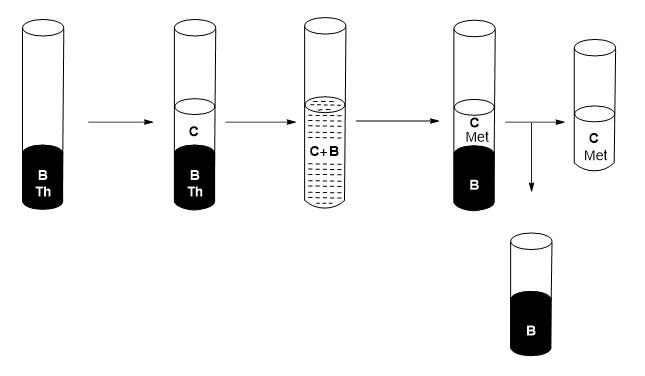
Figure 6: Schematic of the recovery process of the extractant mixture.
TECHNOLOGY ADVANTAGES AND INNOVATIVE ASPECTS
ADVANTAGES OF THE TECHNOLOGY
This novel extraction procedure has the following advantages:
1) It allows the selective extraction of inner transition metals (f-block) from metals belonging to the s-, d- and/or p-blocks of the periodic table in a very efficient way.
2) The extractant mixture is recyclable: once the extraction procedure has been completed, the complexed metal(s) can be fully recovered and the extractant mixture can be used in new extraction cycles.
3) The extractant mixture has a low affinity for the metals of the s-, d- and p- series of the periodic table, so that the metals of these series are extracted with a low or zero percentage.
4) The recovery rate of the extractant mixture is at least 95%, so it is possible to reuse it in new extraction cycles once the extracted metals have been released.
5) The extraction process is environmentally friendly.
6) The procedure is carried out under mild reaction conditions (temperature between 0°C-25°C and atmospheric pressure).
7) Both the TSIL compound and the CYPHOS NTf2solvent are commercially available (or can be easily prepared by simple ion exchange).
8) The procedure is feasible on an industrial scale and can be adapted and implemented to the needs of the company.
In summary, the new extractant mixture is a revolutionary technology that significantly improves on current methods for the extraction of inner transition metals (lanthanides and actinides) in complex matrices.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The present extractant mixture [TSIL + CYPHOS NTf2] has several innovative aspects that differentiate it from other similar technologies on the market. In general, it is not common to combine TSIL compounds with ionic liquids.
Firstly, this novel chemical composition allows the selective and efficient extraction of the inner transition metals of the f-series (lanthanides and actinides) from other metals of the s-, d- and/or p-series of the periodic table. In this sense, the main interaction of the inner transition metals with TSIL, which acts as a selective chelator, takes place via the 1,3-dicarbonyl site.
Moreover, once the extracted metals have been separated, the original extractant mixture is recovered with a yield of more than 95%, which allows it to be recycled and subsequently used in new extraction cycles, making it a sustainable and environmentally friendly procedure (there is no other extraction system of these characteristics on the market that is recyclable).
CURRENT STATE OF DEVELOPMENT
The technology described has been developed on a laboratory scale (state of Technological Readiness Level: TRL = 3).
Tests have shown very promising results. A table with the analyte distribution coefficient (K), which is the ratio of the concentration of the metal in the organic phase to its concentration in the aqueous phase after the extraction procedure, is shown below (see Table 1).
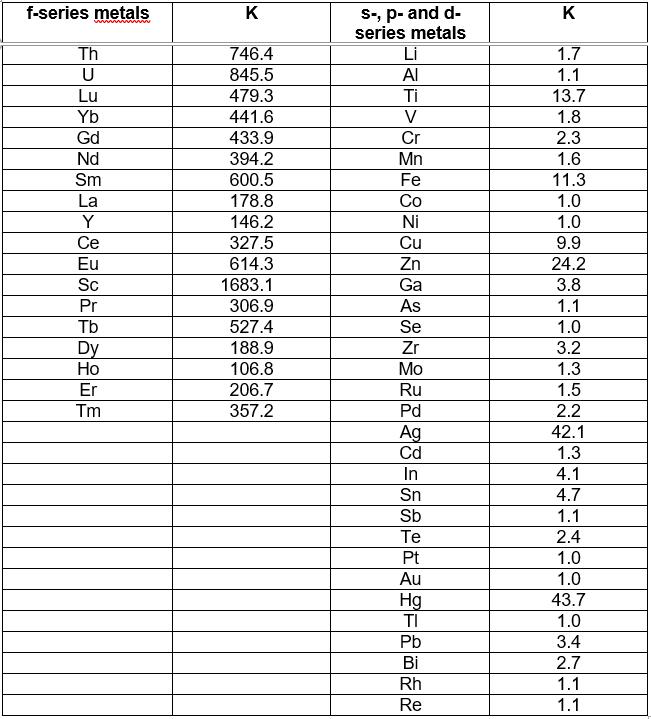
Table 1: The value of the distribution coefficients (K) obtained for the f-series metals, as well as for other metallic elements of the periodic table that can be found in their presence, at pH=6.
These tests have demonstrated the high efficiency and selectivity of the extractant mixture, since the higher the distribution coefficient of the analyte (K), the higher the extraction efficiency.
COLLABORATION SOUGHT
Companies interested in acquiring this technology for commercial exploitation through patent licensing agreements are sought.
Company profile sought:
• Mining.
• Chemical industry.
• Nuclear industry.
• Nuclear medicine.
• Nuclear waste treatment.
TECHNICAL IMAGES (1)

Ahead of the current Coronavirus outbreak, Innoget is fully committed to contributing to mobilizing scientific and expert communities to find a real solution to the Covid-19 pandemic. Therefore, we're supporting worldwide calls and programs that could help in any aspects of the coronavirus crisis.
Is your organization promoting or looking for innovation or research initiatives to mitigate the Covid-19 outbreak? Email us at covid19@innoget.com to list them.
Channeled through Innoget's online open innovation network, initiatives in the health, virology, medicine, or novel technologies applied to human health, among others, are listed and disseminated to Innoget members -ranging from hospitals, research institutes, scientists, businesses, and public administrations- and innovation partners worldwide.
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
Send a request for information
to Universidad de Alicante
Technology Offers on Agri-Food are directly posted
and managed by its members as well as evaluation of requests for information. Agri-Food is the trusted open innovation and science network aimed at directly connect industry needs with professionals online.
Need help requesting additional information or have questions regarding this Technology Offer?
Contact Agri-Food support